Pharma 4.0 with intelligent size changeover
In the pharmaceutical industry, the concept of centerlining is relevant, which means that the optimum machine settings must always be selected in order to prevent unnecessary deviations in the process and thus a reduction in product quality. The aim is to network manufacturing, technology, maintenance and electronic data acquisition in the best possible way in order to make optimum use of plant efficiency. In addition, the growing variety of products and ever smaller batch sizes in pharmaceutical manufacturing demand a high degree of flexibility from the systems.
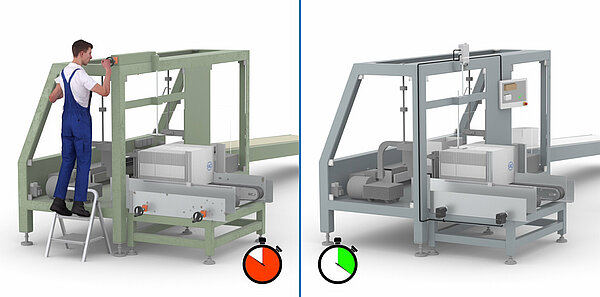
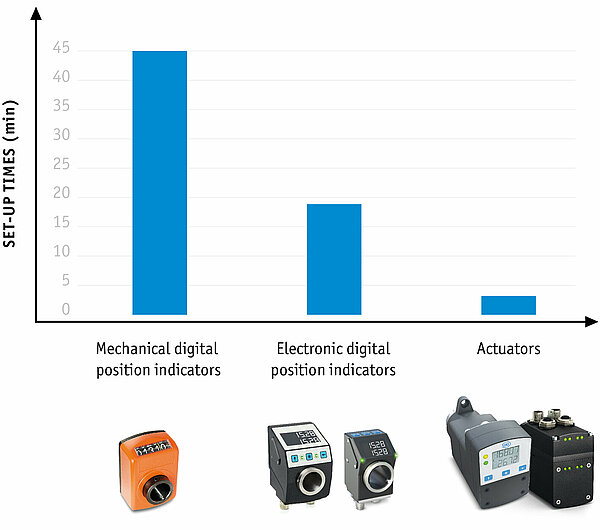
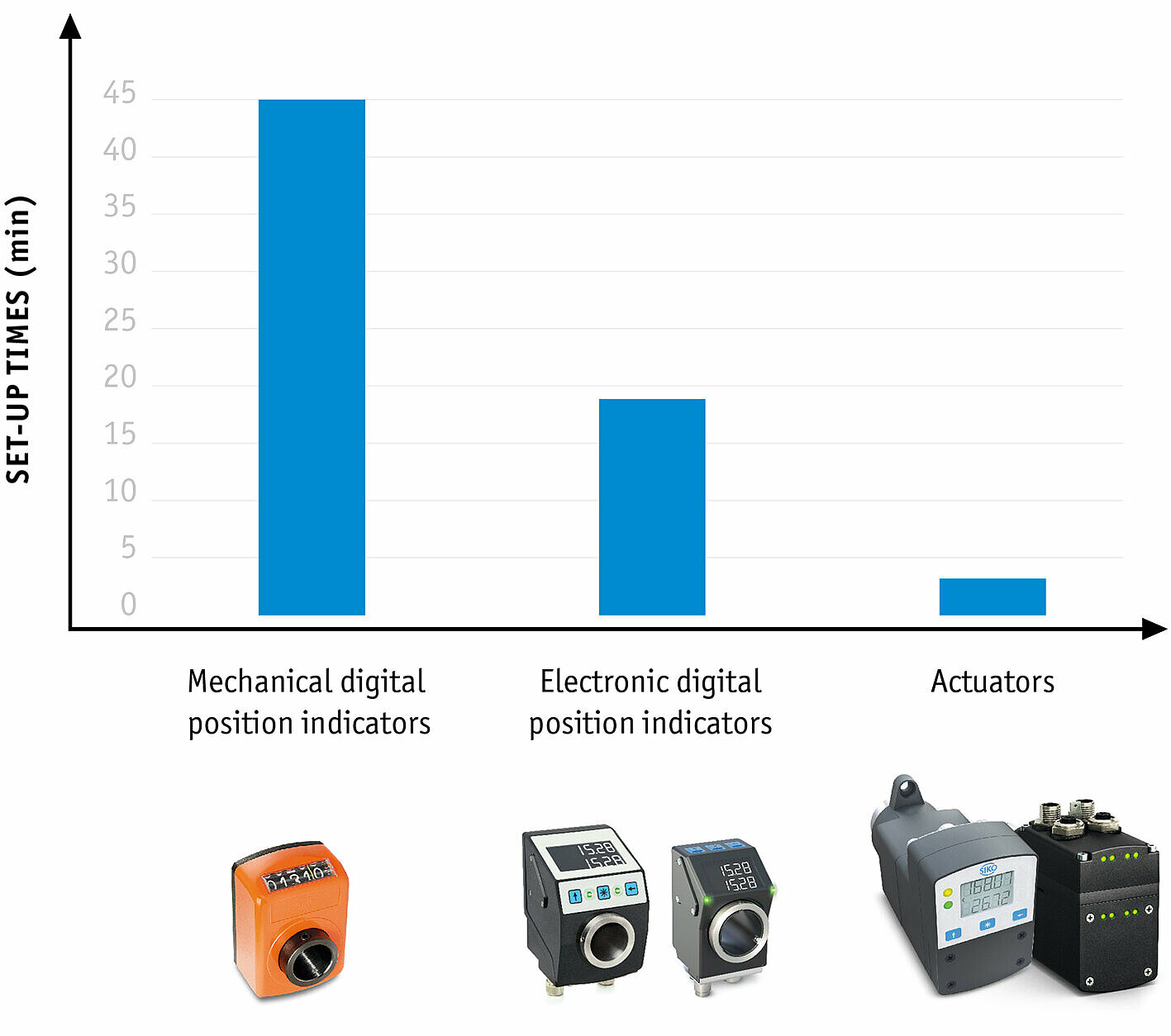
This is where size changeover comes into play, which can be a decisive factor when it comes to system availability. With optimized size changeover, reconfiguration times for product changes can be significantly reduced and process reliability increased. SIKO GmbH, manufacturer of sensors and positioning systems, has introduced various options for size changeover, from purely mechanical position indicators to fully automated positioning drives.
Benefits of optimized size changeover
In pharmaceutical manufacturing, size changeover takes place everywhere, especially in packaging processes, labeling and product inspections. Whenever the dimensions on the machine have to be changed for a new product, this involves size changeovers – whether manually via a crank or automatically via an actuator.
Always exercise caution when changing the machine settings, as errors can creep in and have a negative effect on the product result. Monitored or even automated size changeover minimizes the risk of incorrect settings and can make processes more flexible. The advantages of optimized size changeover are as follows:
- high repeatability, meaning that drugs are always manufactured using the same form and quality
- faster reconfiguration times and thus an increase in process speed
- an increase in efficiency and a reduction in costs
- an increase in process reliability, which is a decisive criterion in pharmaceutical manufacturing
A distinction is made between manual, monitored and automated size changeovers. Which type of format change is most suitable depends on the requirements: the more sizes need to be changed over and the more demanding manufacturing is from a quality perspective – which is usually the case in the pharmaceutical sector – the more sensible it is to use monitored or automated positioning systems.
Manual size changeover
For manual size changeover, both mechanical and electronic position indicators are used, which indicate the actual value of the current position. They are suitable for basic machines with rather infrequent adjustments. The common mechanical-digital SIKO position indicators are very precise, easy to read and are configured specifically for each application.
Electronic position indicators have an advantage over mechanical ones in that they are freely programmable and can therefore be used with greater flexibility. Parameters such as spindle pitch, decimal places, direction of rotation, mounting position or use in angle mode can be conveniently configured. Practical: Mechanical and electronic variants are compatible for installation, so that reconfiguration or expansion is not problematic.
Monitored size changeover
Monitored size changeover is made possible by bus-compatible electronic position indicators with setpoint value specifications, which are integrated into the machine control system. Actual and target values are exchanged and compared between the individual position indicators and the higher-level control unit with the aid of a formula management system in which all product variants are stored with their setpoint values as formulas. This enables increased process reliability, as the system is only restarted when all setpoint and actual values on the indicators match.
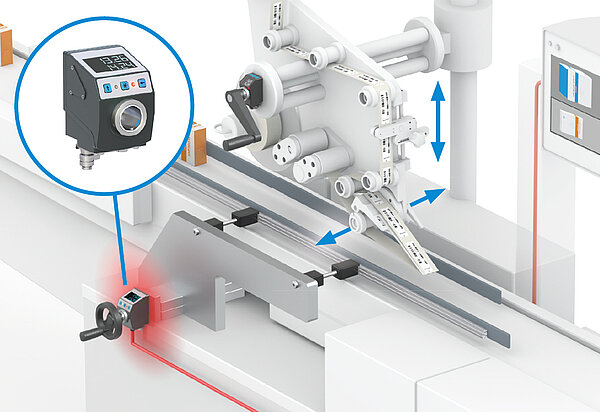
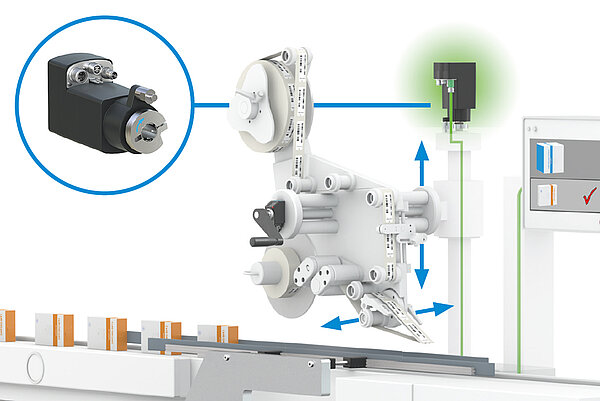
Rejects or damage to system parts are thus avoided. The changeover of the axes is still carried out manually with this variant, whereas the monitoring of the correct settings is carried out electronically. The special feature of the electronic position indicators are the LED lights which clearly display the position status to the operator: Green lights for "position correct", red lights signal "position not correct". In addition, the display includes an integrated arrow direction indicator, which indicates in which direction the changeover has to be made.
Applications for monitored size changeover
Monitored size changeover can be helpful in pharmaceutical manufacturing, for example, in the case of systems for cartoning machines requiring frequent adjustment or in the case of product labeling. A relatively new development is "Track & Trace" systems, which requires prescription drugs to be provided with a clear, traceable label. This involves a wide variety of processes such as printing, reading, labeling and weighing, and thus numerous adjustments. The process reliability of these systems is significantly increased with monitored size changeover.
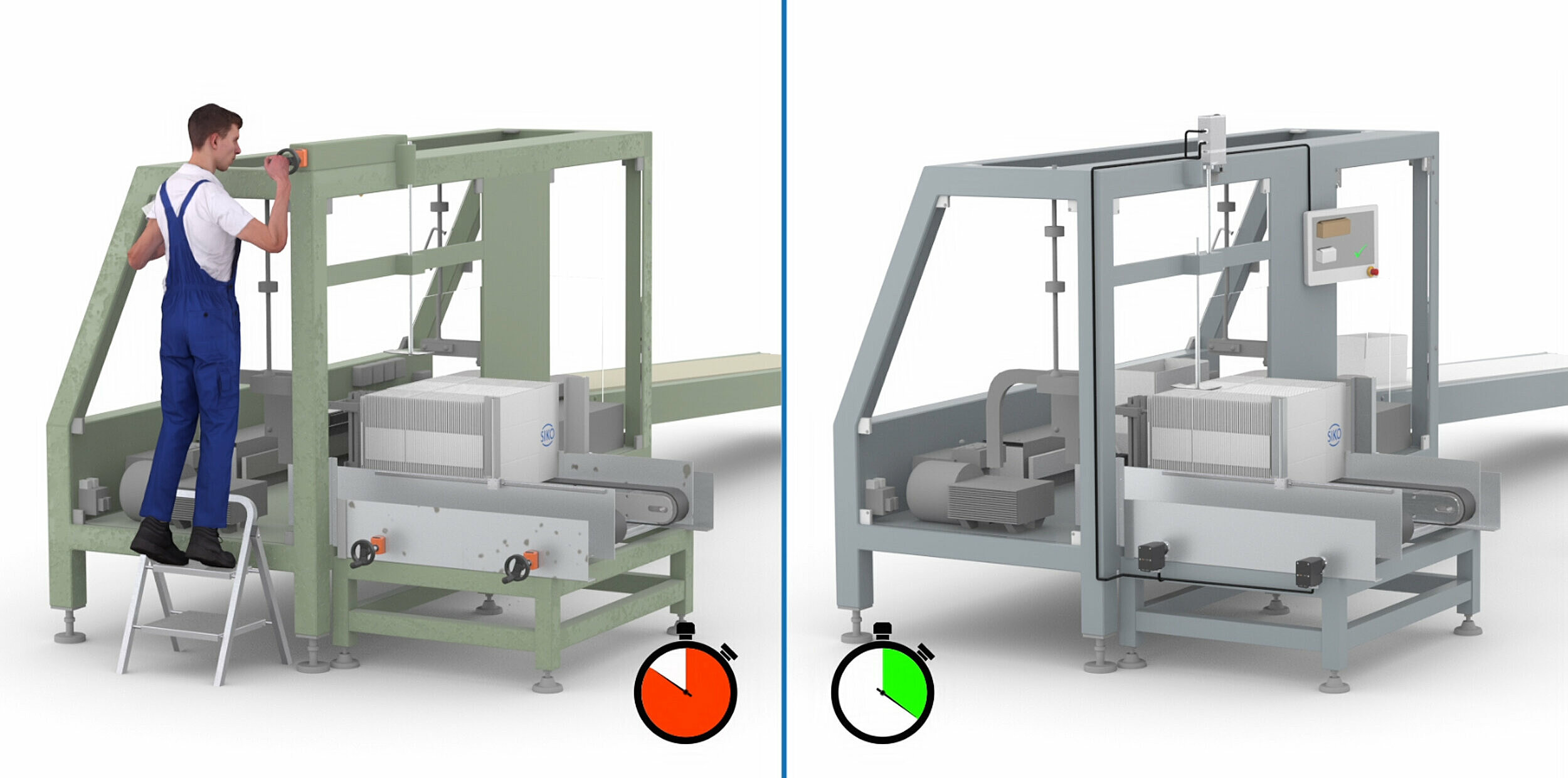
Automatic size changeover
If axes are changed over without any manual intervention, this is referred to as automatic size changeover via compact positioning drives. The actuator, which is networked with the machine control system, moves directly to the required position. With automation, a further significant reduction in reconfiguration times is achieved. In addition, automation is worthwhile if system parts that are difficult to access have to be adjusted frequently.
An actuator is characterized by its highly integrated design, which combines all components in one device: the brushless DC motor (which is wear-free), a low-backlash and powerful gearbox as well as position encoder and power and control electronics. The integration of the actuator into the machine controls as well as the communication with the controls is easily achievable, not least thanks to a large number of common standard interfaces. These include Siemens-compliant Profibus or Profinet interfaces, cost-effective serial interfaces such as RS485 and CAN, IO-Link and modern Industrial Ethernet interfaces.
Depending on the requirements of the application, different power classes of actuators are required: from small actuators with low power requirements for fine adjustment in a folding box magazine to feeding systems for large cardboard boxes where entire machine aggregates have to be moved with actuators with relatively high torques.
With this variant of size changeover, too, the controls only initiate a system restart when the process data exchange between the drive and the control has resulted in a match between the actual and setpoint values.
Predictive Maintenance
Another functionality of the compact actuator makes it possible to draw conclusions about the operating status of both the actuator itself and the system itself: the diagnostic capability. By collecting and monitoring various parameters of the drive, such as current consumption in the motor, temperature or voltage values at the control and load circuit, irregularities and thus the need for maintenance can be detected at an early stage. If specified values are exceeded, measures can be taken immediately.
Conclusion: More automation, more data
In pharmaceutical manufacturing, smart size changeover involves several dimensions: there are the directly measurable effects such as reduction of set-up times, increased plant availability, increased efficiency and increased process reliability. In addition, intelligent solutions help to carry out such secondary processes within manufacturing as unobtrusively as possible. If possible, you should not have to worry about size changeover; if it works well and reliably, you can turn your attention to key demanding activities such as line clearance or extensive documentation.
Peripheral components such as position indicators and servomotors are becoming increasingly important as a result of increasing automation and digitalization. This is demonstrated by recent developments such as "Track & Trace" systems. Additional benefits such as data collection, self-diagnosis and service life monitoring of the components make smart size changeover a crucial element of Pharma 4.0.